
李成杰 (1984. 12),副教授 (博士生导师)
联系方式:电话 021-64253530、邮箱 chengjie.li@ecust.edu.cn、徐汇校区实验十三楼-110A
研究方向:基于吡咯周环反应高效创制新颖共轭体系并探索相关应用
招生专业:应用化学 (硕士、博士)、材料与化工 (专业硕士)
教育经历:2003.09-2012.06天津大学 (本、硕、博) 导师:冯亚青 教授
工作经历:2012.10-2016.10奥地利因斯布鲁克大学 博士后 导师:B. Kräutler 教授
2016.12-2018.08华东理工大学 化学与分子工程学院 助理研究员
2018.09-至今华东理工大学 化学与分子工程学院 副教授
项目情况
4. 国家自然科学基金委员会面上项目 22075077,新型缺电子芳香稠环卟啉分子的合成与性质研究 (2021-01 至 2024-12、在研、主持)
3. 上海市科学技术委员会面上项目 20ZR1414100,基于环加成反应构建新型缺电子芳香稠环卟啉 (2020-07 至 2023-06、在研、主持)
2. 国家自然科学基金委员会青年项目 21702062,新型稠环BODIPY近红外生物硫醇荧光探针的研究 (2018-01 至 2020-12、结题、主持)
1. 上海市科学技术委员会上海市浦江人才计划17PJ1401700,基于BODIPY和卟啉的生物硫醇荧光探针研究 (2017-07 至 2019-06、结题、主持)
发表论文详情
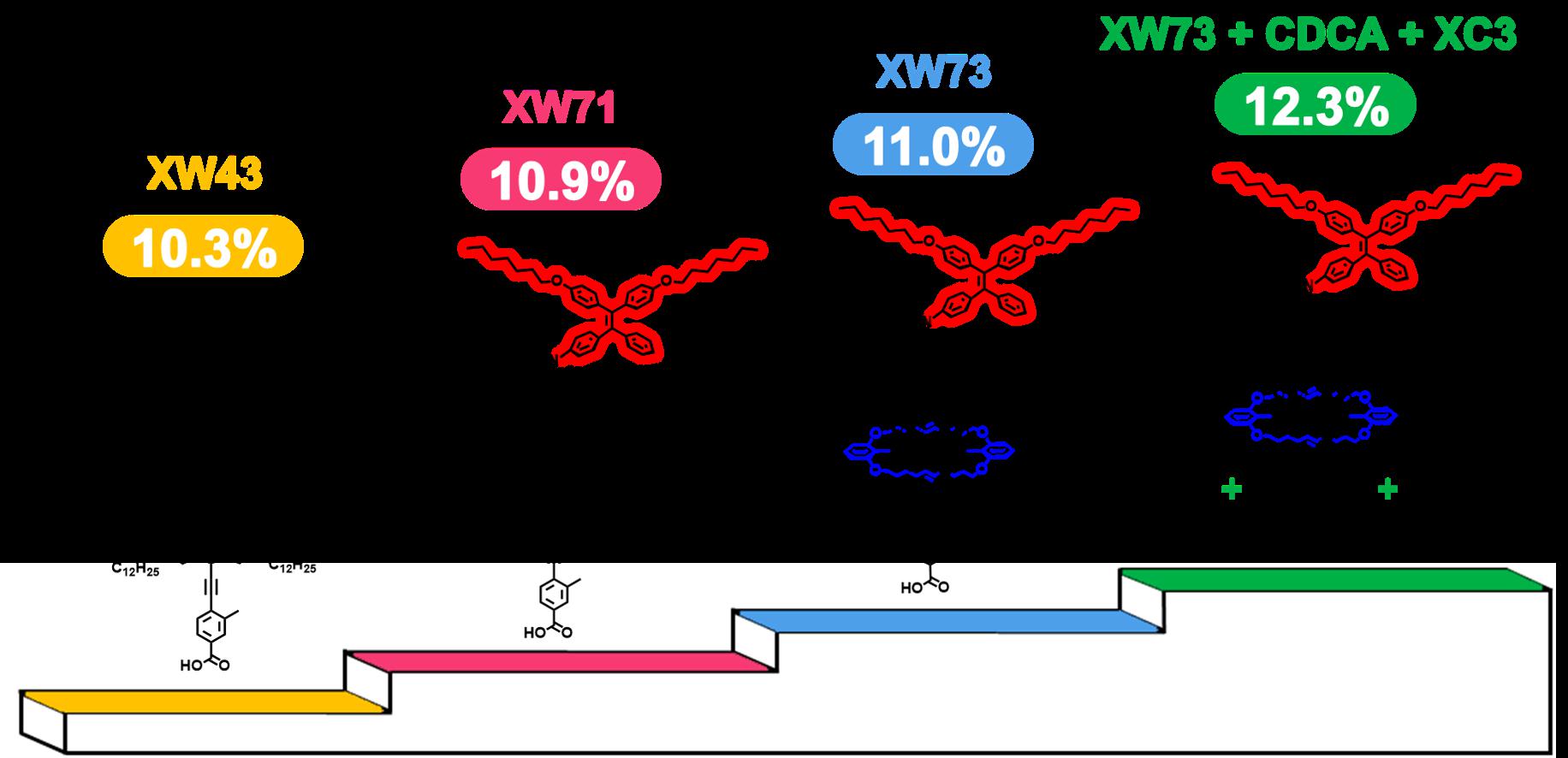
[32]Zou, J.#; Wang, Y.#; Baryshnikov, G.; Luo, J.; Wang, X.; Ågren, H.; Li, C.*; Xie, Y.* Efficient dye sensitized solar cells based on a new class of doubly concerted companion dyes, ACS Applied Materials & Interfaces, 2022, 14(29): 33274-33284.
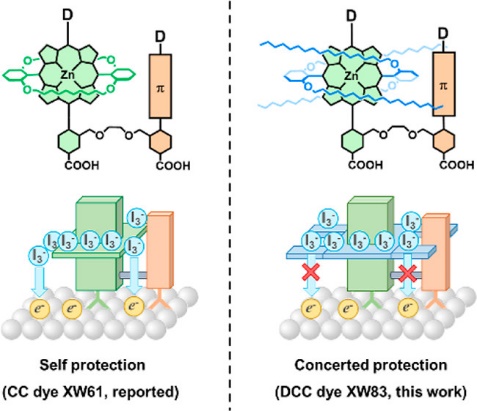
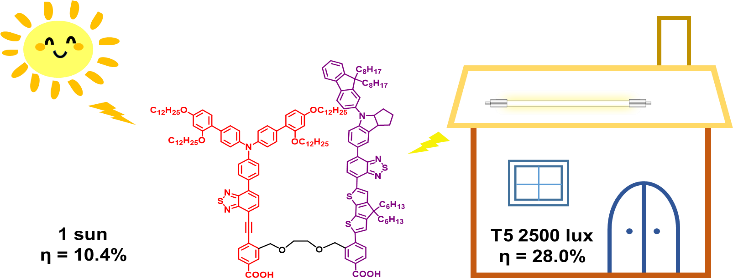
[31]Wang, X.; Wang, Y.; Zou, J.; Luo, J.; Li, C.*; Xie, Y.* Efficient solar cells sensitized by organic concerted companion dyes suitable for indoor lamps, ChemSusChem, 2022, e202201116.
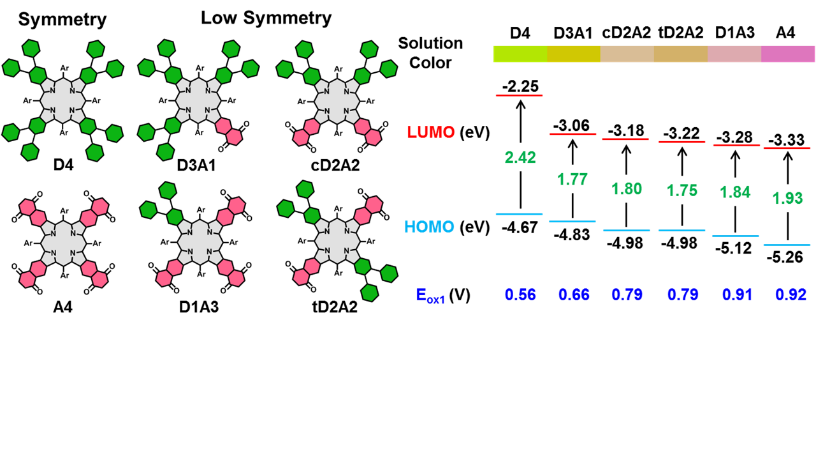
[30]Cao, G.; Baryshnikov, G.; Chen, C.; Chen, L.; Zhao, T.; Fu, S.; Jiang, Z.; Liu, X.;* Li, Q.; Xie, Y.; Li, C.* Porphyrindiene-based tandem Diels-Alder reaction for preparing low-symmetry π-extended porphyrins with push-pull skeletons. The Journal of Organic Chemistry, 2022, 87(14): 9001-9010.
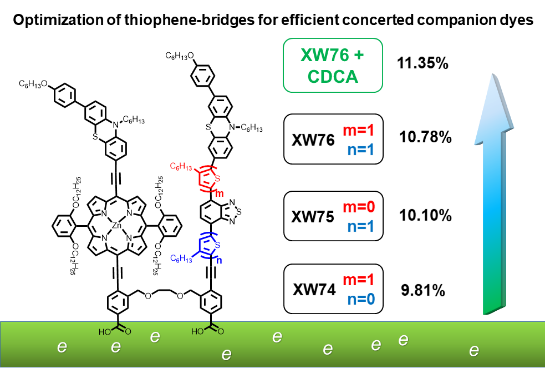
[29]Luo, J.; Xie, Z.; Zou, J.; Wu, X.*; Gong, X.*; Li, C.*; Xie, Y.* Efficient dye-sensitized solar cells based on concerted companion dyes: Systematic optimization of thiophene units in the organic dye components. Chinese Chemical Letters, 2022, 33(9): 4313-4316.
[28]Li, C.; Kräutler, B.* Facile retro-Dieckmann cleavage of a pink phyllobilin: new type of potential downstream steps of natural chlorophyll breakdown. Monatshefte für Chemie - Chemical Monthly, 2022, doi.org/10.1007/s00706-022-02894-z
[27]Zou, J.; Tnag, Y.; Baryshnikov, G.; Yang, Z.; Mao, R.; Feng, W.; Guan, J.; Li, C.*; Xie, Y.* Porphyrins containing a tetraphenylethylene-substituted pheno-thiazine donor for fabricating efficient dye sensitized solar cells with high photovoltages. Journal of Materials Chemistry A, 2022, 10(3): 1320-1328.
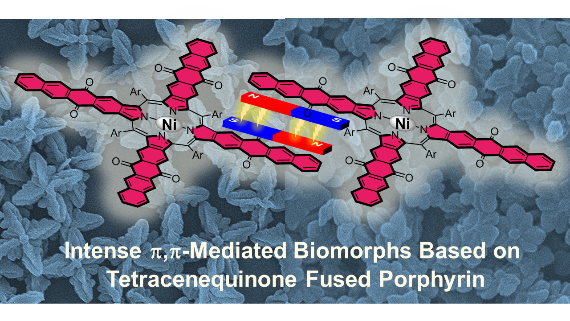
[26]Chen, C.; Li, D.; Cao, G.; Qin, Z.; Xu, Y.; Liu, X.*; Li, Q.; Xie, Y.; Li, C.* Solvent-regulated biomorphs from the intense ,-mediated assemblies of tetracenequinonefused porphyrin. CrystEngComm, 2021, 23(43): 7565-7569. (Cover story)

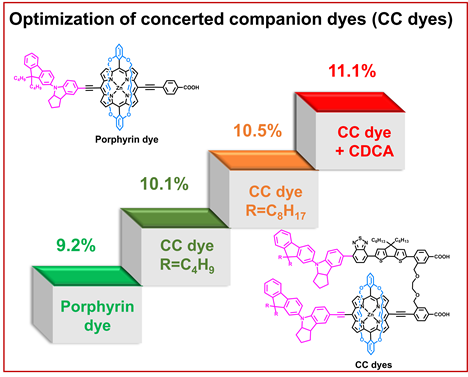
[25]Chen, Y.#; Tang, Y.#; Zou, J.; Zeng, K.; Baryshnikov, G.; Li, C.*; Xie, Y.* Fluorenyl indoline as an efficient electron donor for concerted companion dyes: Enhanced light-harvesting and photocurrent. ACS Applied Materials & Interfaces, 2021, 13(42): 49828-49839.
[24]Li, C.; Podewitz, M.; Kräutler, B.* A blue zinc complex of a dioxobilin-type pink chlorophyll catabolite exhibiting bright chelation-enhanced red fluorescence. Eurpean Journal of Inorganic Chemtry, 2021, 2021(20): 1904-1912. (Cover story)

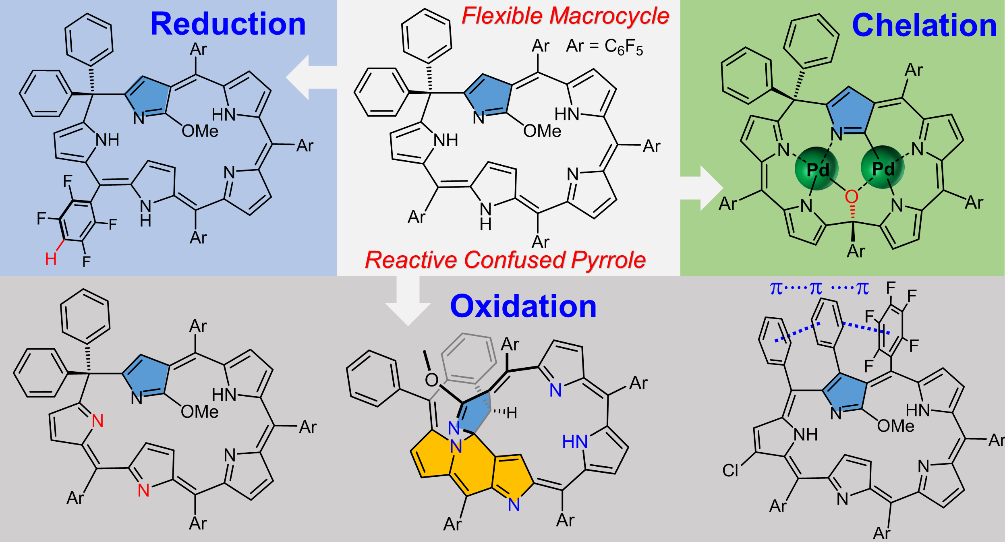
[23]Li, C.#; Li, Q.#; Shao, J.; Tong, Z.; Ishida, M.; Baryshnikov, G.; Ågren, H.; Furuta, H.*; Xie, Y.* Expanded N-confused phlorin: A platform for a multiply fused polycyclic ring system via oxidation within the macrocycle. Journal of the American Chemical Society, 2020, 142(40): 17195-17205.
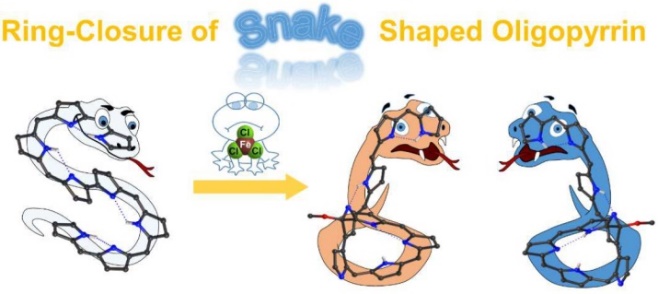
[22]Li, C.#; Zhang, K.#; Ishida, M.; Li, Q. Z.; Shimomura, K.; Baryshnikov, G.; Li, X.; Savage, M.; Wu, X.-Y.; Yang, S. H.; Furuta, H.*; Xie, Y.* Tripyrrin-armed isosmaragdyrins: synthesis, heterodinuclear coordination, and protonation-triggered helical inversion. Chemical Science, 2020, 11(10): 2790-2795.
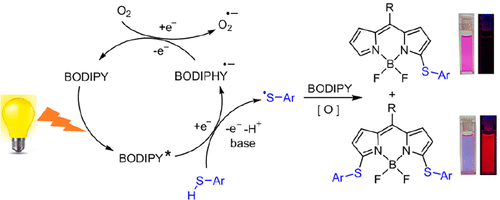
[21]Ma, F.; Zhou, L.; Liu, Q.; Li, C.*; Xie, Y.* Selective photocatalysis approach for introducing ArS units into BODIPYs through thiyl radicals. Organic Letters, 2019, 21(3): 733-736.
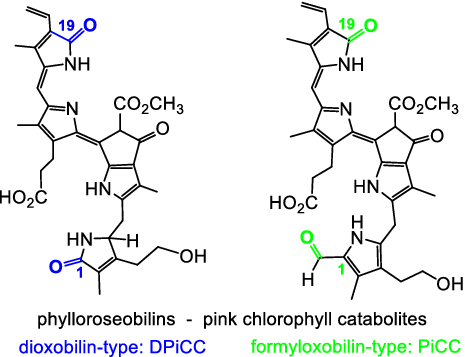
[20]Li, C.; Erhart, T.; Liu, X.; Kräutler, B.* Yellow dioxobilin-type tetrapyrroles from chlorophyll breakdown in higher plants-A new class of colored phyllobilins. Chemistry-a European Journal, 2019, 25(16): 4052-4057.

[19]Li, C.; Kräutler, B.* A pink colored dioxobilin-type phyllobilin from breakdown of chlorophyll. Monatshefte Für Chemie - Chemical Monthly, 2019, 150(5): 813-820.
[18]Shao, J.; Li, C.*; Kong, J.; Jiang, H.; Zhao, S.; Li, M.; Liang, X.; Zhu, W.; Xie, Y.* Regioselective oxidative ring cleavage of a phlorin analogue: An approach for synthesizing linear tetrapyrroles. Organic Letters, 2018, 20(7): 1941-1944.
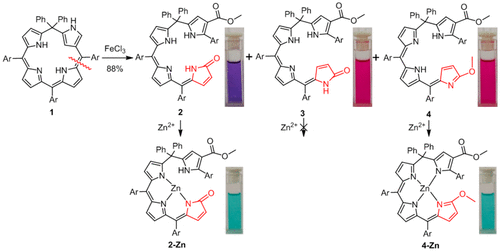
[17]Li, C.; Wurst, K.; Kräutler, B.* A dipyrrin programmed for covalent loading with fullerenes. Chemistry-a European Journal, 2018, 24(40): 10032-10037.
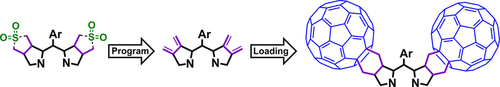
[16]Li, C.; Wurst, K.; Berghold, J.; Podewitz, M.; Liedl, K. R.; Kräutler, B.* Pyro-phyllobilins: elusive chlorophyll catabolites lacking a critical carboxylate function of the natural chlorophylls. Chemistry-a European Journal, 2018, 24(12): 2987-2998.
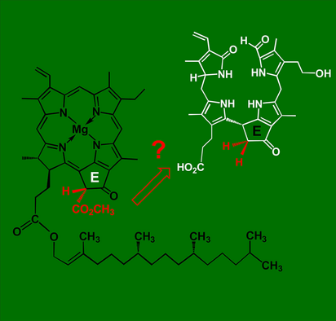
[15]Erhart, T.#; Mittelberger, C.#; Liu, X.#; Podewitz, M.#; Li, C.#; Scherzer, G.; Stoll, G.; Valls, J.; Robatscher, P.; Liedl, K. R.; Oberhuber, M.; Kräutler, B.* Novel types of hypermodified fluorescent phyllobilins from breakdown of chlorophyll in senescent leaves of grapevine (Vitis vinifera). Chemistry-a European Journal, 2018, 24(65): 17268-17279.
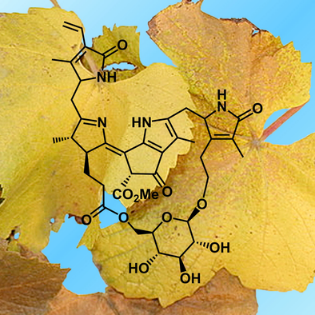
[14]Li, C.; Zhang, J.; Song, J.; Xie, Y.*; Jiang, J. Synthetic porphyrin chemistry in China. Science China-Chemistry, 2018, 61(5): 511-514.
[13]Wang, Q.; Wei, X.; Li, C.*; Xie, Y.* A novel p-aminophenylthio- and cyano-substituted BODIPY as a fluorescence turn-on probe for distinguishing cysteine and homocysteine from glutathione. Dyes and Pigments, 2018, 148: 212-218.
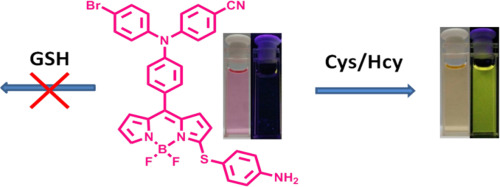
[12]Wang, Q.; Ma, F.; Tang, W.; Zhao, S.; Li, C.*; Xie, Y.* A novel nitroethylene-based porphyrin as a NIR fluorescence turn-on probe for biothiols based on the Michael addition reaction. Dyes and Pigments, 2018, 148: 437-443.
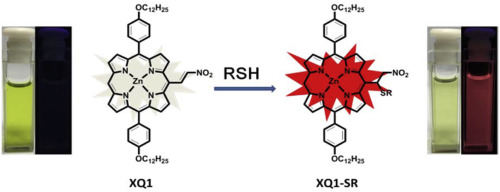
[11]Li, C.; Liu, X.; Shao, J.; Su, G.; Xie, Y.* Synthesis of a doubly SO2-fused phlorin: Tuning the structure and properties by the SO2 groups. Journal of Porphyrins and Phthalocyanines, 2018, 22(9-10): 799-806.

[10]Kong, J.; Shao, J.; Li, C.*; Qi, D.; Li, M.; Liang, X.; Zhu, W.; Jiang, J.; Xie, Y.* Neo-N-confused phlorins and phlorinone: rational synthesis and tunable properties. Organic Letters, 2017, 19(3): 650-653.
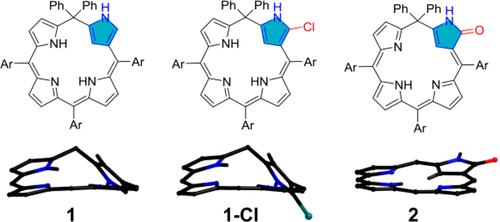
[9]Li, C.; Wurst, K.; Jockusch, S.; Gruber, K.; Podewitz, M.; Liedl, K. R.; Kräutler, B.* Chlorophyll-derived yellow phyllobilins of higher plants as medium-responsive chiral photoswitches. Angewandte Chemie-International Edition, 2016, 55(51): 15760-15765. (Inside cover story)

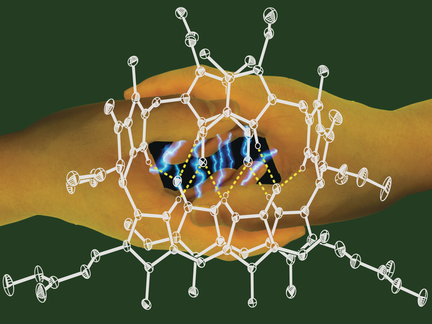
[8]Li, C.; Kräutler, B.* Zn-complex of a natural yellow chlorophyll catabolite. Journal of Porphyrins and Phthalocyanines, 2016, 20(1-4): 388-396.
[7]Li, C.; Wurst, K.; Feng, Y.; Kräutler, B.* Synthesis, spectroscopic and crystallographic analysis of the Zn-complex of a di(beta,beta'-sulfoleno)pyrrin: model for Zn-complexes of bilirubin and of phylloxanthobilins. Monatshefte Für Chemie, 2016, 147(6): 1031-1036.
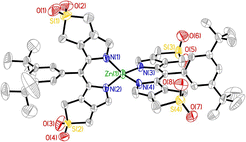
[6]Li, C.; Kräutler, B.* Transition metal complexes of phyllobilins-A new realm of bioinorganic chemistry. Dalton Transactions, 2015, 44(22): 10116-10127.

[5]Li, C.; Zhang, J.; Liu, X.; Zhou, Y.; Sun, D.; Cheng, P.; Zhang, B.; Feng, Y.* Synthesis of corrole-fullerene dyads via [4+2] cycloaddition reaction. RSC Advances, 2014, 4(77): 40758-40762.
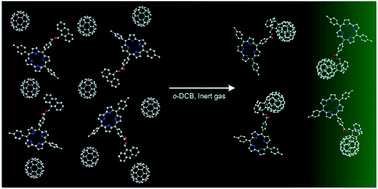
[4]Li, C.; Ulrich, M.; Liu, X.; Wurst, K.; Müller, T.; Kräutler, B.* Blue transition metal complexes of a natural bilin-type chlorophyll catabolite. Chemical Science, 2014, 5(9): 3388-3395.

[3]Li, C.; Zhang, T.; Zeng, Z.; Liu, X.; Zhao, Y.; Zhang, B.; Feng, Y.* A New Route to Indazolone via Amidation Reaction of o-Carboxyazobenzene. Organic Letters, 2012, 14(2): 479-481.
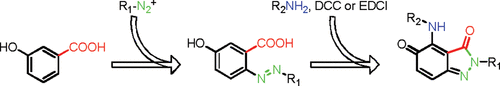
[2]Li, C.; Fechtel, M.; Feng, Y.; Kräutler, B.* Corroles programmed for regioselective cycloaddition chemistry-Synthesis of a bisadduct with C60-fullerene. Journal of Porphyrins and Phthalocyanines, 2012, 16(5-6): 556-563.
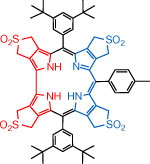
[1]Li, C.; Feng, Y.*; Liu, X.; Zhang, T. The synthesis of porphyrin-anthraquinone dyad via an azo-rearrangement. Chinese Chemical Letters, 2011, 22: 539-542.