Recently, He Tian’s group has made a new breakthrough in “Vibration Induced Emission (VIE)”, and a paper titled “Snapshotting the Excited-State Planarization of Chemically-Locked N,N’-Disubstituted-dihydrodibenzo[a,c]phenazines” has been published on J. Am. Chem. Soc. (http://pubs.acs.org/doi/pdfplus/10.1021/jacs.6b11789).
“Vibration Induced Emission” (VIE) is a new fluorescent mechanism, which was proposed by Prof. Tian to rationalize the anomalously large Stokes Shift and dual emission from the N,N'-disubsitituted-dihydrophenazines. At present Tian’s group has obtained significant progress and published a series of papers about this new mechanism: firstly, they observed dihydrophenazine-derivant exhibit remarkable large Stokes Shift and dual emission in solution, which could be ascribe to the excited-state configuration transformations induced by vibration of molecules, and then they named this new mechanism as Vibration Induced Emission (VIE) (Chem. Commun., 2015, 51,4462); With a collaboration with Prof. Pi-Tai Chou in National Taiwan University, VIE was validated by temperature dependent steady-state spectroscopy, nanosecond time-resolved spectroscopy, femtosecond dynamics and computational simulation of the reaction energy surfaces afterwards (J. Am. Chem. Soc., 2015, 137, 8509); They also designed and synthesized a phenazine-based ratiometric fluorescent probe for recognizing Hg2+ based on VIE (Small, 2016, 12, 6542); Besides a water-soluble VIEgen was designed and utilized to image amyloid b fibrils, which are a signature of neurological disorders such Alzheimer’s disease (Faraday Discuss., DOI: 10.1039/C6FD00156D); Additionally, the synthetic mechanism of dihydrophenazines was systematically studied as well (Chem. Commun., 2016, 52, 5459).
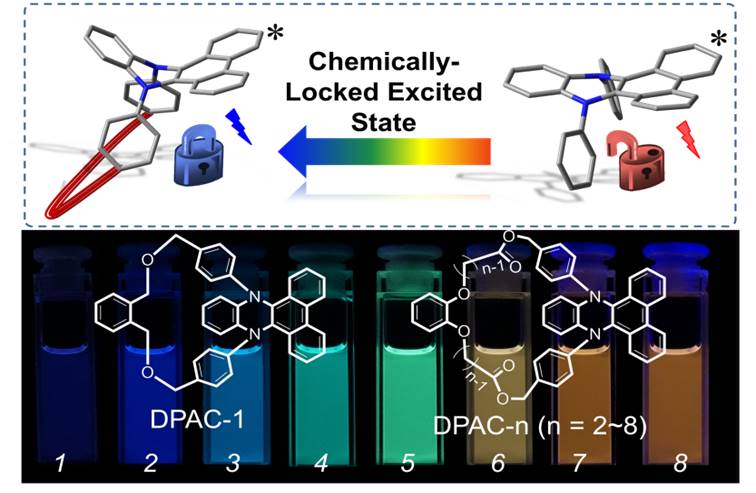
For deeper understanding of VIE mechanism, Tian’s group conceived a series of annulated structures based on dihydrophenazine. Their aim was to impose various degrees of constraint to impede the excited-state structural vibration in hope of controlling the excited-state planarization of the molecules (as shown in figure below). In this study, a series of DPAC-n (n=1-8) derivatives were synthesized, in which n correlates with the alkyl length, such that the strength of spatial constraint increases as n decreases. With the degree of constraint increasing, the probability of excited-state planarization reduced, resulting in a blue shift. As a result, drastic chain-length dependent emission was observed, spanning much of the panchromatic region. For elaborating the mechanism, they once again cooperated with Prof. Chou. Detailed spectroscopic and dynamic studies, together with computational approach, firmly supported the harness of planarization by imposing spatial constraint in DPAC-n, which stepped on a brake during the structure evolution in the excited state. Moreover, severing the linkagefor e.g., DAPC-5by base treatment could release the structure constraint and hence resulted in drastic changes of emission from blue (400 nm) to red (610 nm), demonstrating the perspective as well as versatility for the use of sensing elements.
This work was mainly completed by Ph.D. candidate Wei Chen (ECUST), Ph.D. candidate Chi-Lin Chen (NTU), post-doctor Zhiyun Zhang (NTU), et al., and supported by National 973 Program, NSFC for Creative Research Groups.




