Recently, Prof. He Tian, Prof. Jianhua Su’s group, and Pi-Tai Chou’s group achieved remarkable advance in the luminescence mechanism of conjugated molecules. A paper entitled “Excited-State Conformational/Electronic Responses of Saddle Shaped N, N-Disubstituted- Dihydrodibenzo [a, c] phenazines: Wide Tuning Emission from Red to Deep Blue and White Light Combination” was published in J. Am. Chem. Soc.( 2015, 137, 8509-8520).
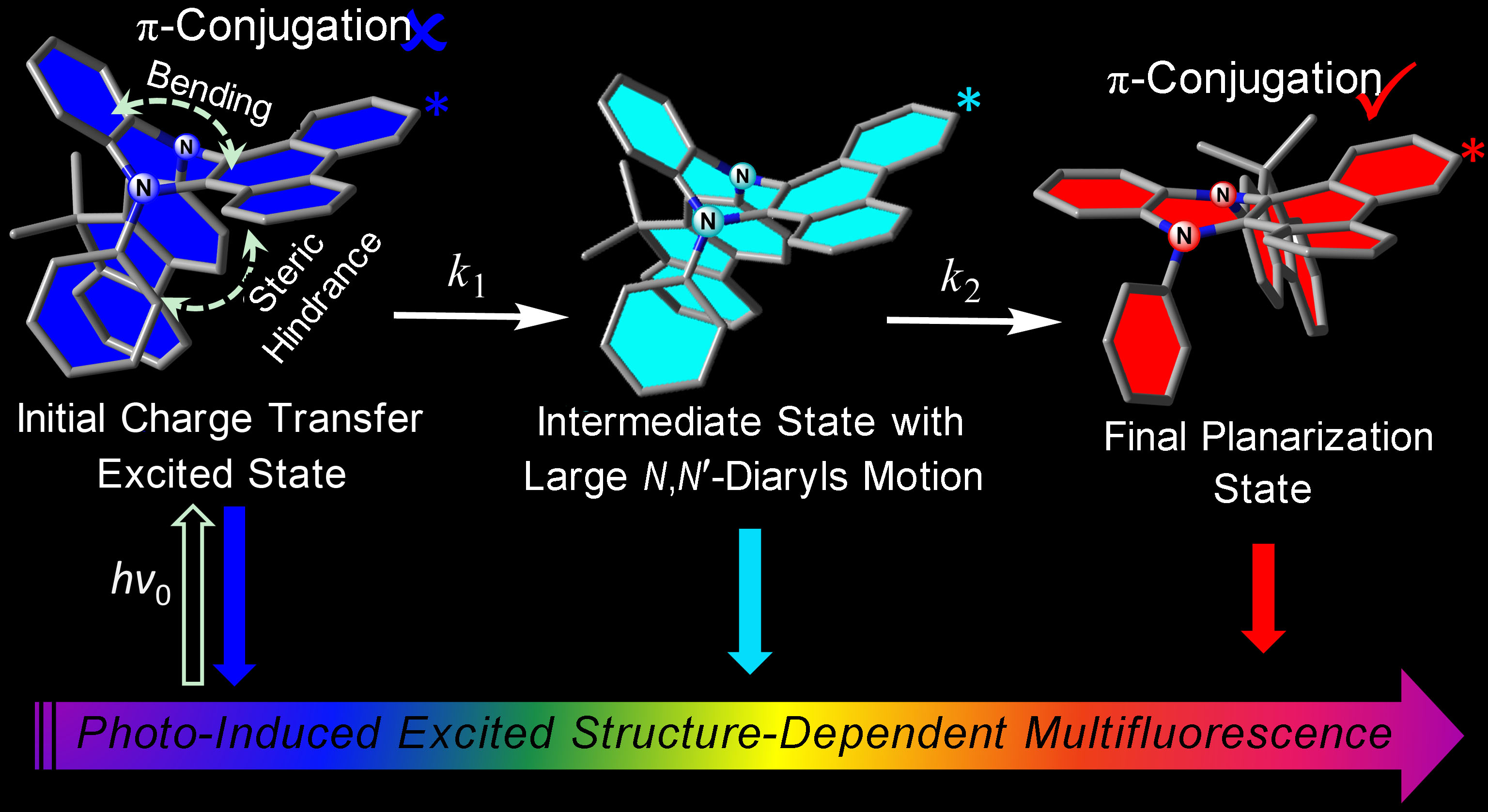
Shedding light on the excited-state dynamics of luminophores relevant to anomalous Stokes shift, multiple emissions, and environmental effects is a long-standing challenge.Molecules having distinct differences in electronic configuration and/or structures between electronically ground and excited states may display very distinct photophysical properties, such as large Stokes-shifted and/or multiple emissions, which are of both fundamental and application interests. Recently, Prof. Jianhua Su, Doctor Zhiyun Zhang, and Doctor Wei Huang, et al., observed a single organic molecule, phenazine-derivant,, exhibit two fluorescent peaks in solution. They then controlled the vibrations of the molecules, finally obtaining a color-tunable with white emission process(W. Huang, et al., Chem. Commun. 2015, 51, 4462-4464). Therefore, they proposed a new mechanism named “vibration induced emission”. In order to validate the theory, they cooperated with Prof. Pi-Tai Chou in Taiwan. Doctor Zhiyun Zhang even went to Taipei Imperial University to better study with the spectrum scholars. With three years in-depth research, the results have got the acceptance in the academic field. A series of saddle-shaped N,N- disubstituted- dihydrodibenzo [a,c] phenazine donor-acceptor dyads have been reported in J. Am. Chem. Soc.. They revealed remarkably large Stokes-shifted emission that was independent of the solvent polarity. These unusual and unique photophysical behaviors were rationalized by electronic configuration coupled conformation changes en route to the geometry planarization in the excited state. This proposed mechanism was firmly supported by polarity, viscosity, and temperature dependent steady-state and nanosecond time-resolved spectroscopy. Together with femtosecond early dynamics and computational simulation of the reaction energy surfaces, the results lead they to establish a sequential, three-step kinetics. Upon electronic excitation of these saddle-shaped N,N-disubstituted phenazines, intramolecular charge transfer took place, followed by the combination of polarization stabilization and skeletal motion toward the planarization, i.e., elongation of the p-delocalization over the benzo[a,c]phenazines moiety. Along the planarization, DPAC and FlPAC encountered steric hindrance raised by the N,N-disubstituted side chain, resulting in a local minimum state, i.e., the intermediate. The combination of initial charge transfer state, intermediate, and the final planarization state rendered the full spectrum of interest and significance in their anomalous photophysics. Depending on rigidity and polarity, the titled N,N-disubstituted phenazines exhibited multiple emissions, which can be widely tuned from red to deep blue and even to white light generation upon optimization of the surrounding media. The associated geometry relaxed phenomena of these titled phenazines thus provided a new paradigm en route to versatile lighting materials, more dyes prepared according to the new mechanism “vibration induced emission” is under research.
This work was mainly completed by Zhiyun Zhang, et al., and supported by National 973 Program, NSFC for Creative Research Groups, et al.




