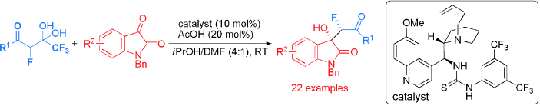
Great progress has been recently made by Dr. Fang Xiang and Ph. D student Ibrayim Saidalimu on fluorinated asymmetric catalysis, which has been published on Angew. Chem. Int. Ed. (“Highly Enantioselective Construction of 3-Hydroxy Oxindoles through a Decarboxylative Aldol Addition of Trifluoromethyl a-Fluorinated gem-Diols to N-Benzyl Isatins” DOI: 10.1002/anie.201301443).
Asymmetric fluorination reaction is one of the research hot spot in organic fluorine chemistry. Though a lot of works have been published in substrate-controlled enantioselectivity, reactions on constructing monofluorinated chiral center by selective C-C cleavage are seldomly reported. In this research work, a new and efficient direct aldol reaction of gem-diols to isatins catalyzed by bifunctional thiourea derived from a cinchona alkaloid was developed. The key step of this protocol is the release of trifluoroacetate for the cleavage of a C-C bond by making use of gem-diols as a synthetic equivalent of fluorinated aryl/alkyl methyl ketone enolates. The resulting decarboxylated products were obtained almost quantitatively with excellent selectivity. This strategy could complement the existing asymmetric fluorinations, thereby facilitating the synthesis of chiral fluorinated molecules for medicinal development.
This work is supported by the National Natural Science Foundation of China and Science and Technology Commission of Shanghai Municipality. This project was successfully carried out by Associated Prof. Fang Xiang and Ph. D student Ibrayim Saidalimu with the help and support from Prof. Wu Xinyan.