Recently, Prof. Shi Min group reported two works which were《Rhodium(II)-catalyzed intramolecular annulation of 1-sulfonyl-1,2,3-triazoles: facile synthesis of N-bridgehead azepine skeletons》(Angew. Chem. Int. Ed. 2014, 53, 5142-5146; http://onlinelibrary.wiley.com/doi/10.1002/anie.201400881/abstract) and《RhII-catalyzed intramolecular cycloisomerizations of methylenecyclopropanes with N-sulfonyl 1,2,3-triazoles》(Angew. Chem. Int. Ed. 2014, DOI: 10.1002/anie.201402803; http://onlinelibrary.wiley.com/doi/10.1002/anie.201402803/abstract).
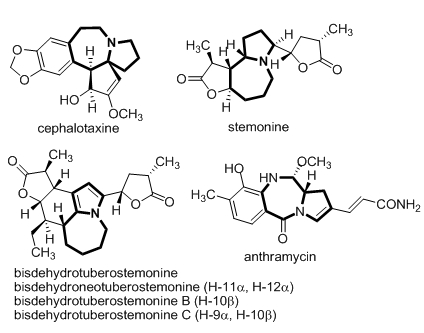
Click chemistry is a term applied to chemical synthesis tailored to generate substances quickly and reliably by joining small units together. Click chemistry is not a single specific reaction, but describes a way of generating products that follows examples in nature, which also generates substances by joining small modular units. N-sulfonyl-1,2,3-triazoles, which can be easily prepared from copper(I)-catalyzed azide-alkyne cycloaddition (CuAAC) have recently received much attention. And the azepine skeleton is a privileged structural motif in many biologically active and medicinally valuable molecules (Figure 1). Compared to five- or six-membered ring systems, the synthesis of seven-membered ring systems such as azepine skeleton still represents a great challenge for synthetic organic chemists.
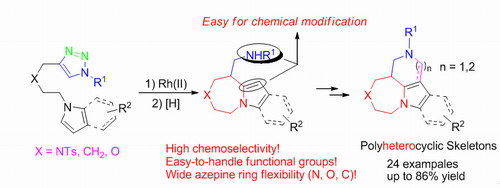
Based on their previous work of gold-catalyzed cycloisomerization of 1,6-diynes (Angew. Chem. Int. Ed. 2011, 50, 2583-2587) and 1,1-bis(indolyl)-5-alkynes (Angew. Chem. Int. Ed. 2013, 52, 6767-6771), the Prof. Shi group successfully prepared stable pyrrolyl and indolyl triazoles through copper(I)-catalyzed azide-alkyne cycloaddition (CuAAC) and investigated their performance in Rh(II) catalyzed annulation reactions. A convenient and efficient synthetic method has been developed to construct highly functionalized N-bridgehead azepine skeletons, which are of great importance in biological and pharmaceutical industry. The reaction proceeded through rhodium(II) azavinyl carbene intermediate which then initiated the intramolecular C-H functionalization with pyrrolyl and indolyl rings. A variety of azepine derivatives were obtained in moderate to good yields under mild conditions in high chemoselectivity along with wide azepine ring flexibility (N, O, C). Several interesting derivatizations of the product well demonstrate that our method is synthetically valuable and useful. Jin-Ming Yang, a Ph.D student of our college, is the first author of this work.
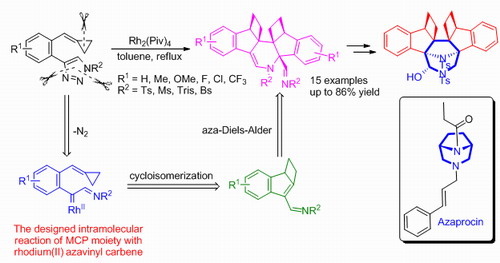
In addition,based on their previous work of Lewis or Brønsted acid-mediated or gold-catalyzed tandem reactions of methylenecyclopropanes (Acc. Chem. Res. 2012, 45, 641-652; Acc. Chem. Res. 2014, 47, 913-924), the Prof. Shi group reported RhII-catalyzed novel tandem cycloisomerizations of methylenecyclopropanes (MCPs) with N-sulfonyl 1,2,3-triazoles, producing a series of highly functionalized polycyclic N-heterocycles via rhodium-imino carbene intermediate. A distinct feature of this divergent synthesis is that different types of substrates control the reaction pathways. Moreover, several interesting transformations of these products to construct diazabicyclo[3.2.1]octane derivatives have been also reported. PhD student Kai Chen mainly completed this work.






